R.E. Kambic*, T.J. Roberts, and S.M. Gatesy
*Author for correspondence: Robert Kambic | Published articles
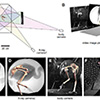
 Our current understanding of avian bipedalism is deeply rooted in a 2-D paradigm. Kinematic and kinetic analyses are typically limited to flexion/extension, but birds and other so-called 'erect' tetrapods must routinely operate outside of the parasagittal plane. We are using marker-based XROMM to measure the 3-D motion and forces/moments in a chicken-like bird, the Helmeted Guineafowl (Numida meleagris), during maneuvering and steady locomotion (Figure 1). We are focusing on long-axis rotation (LAR) because soft tissue artifacts have previously prevented accurate measurement of this degree of freedom. Rather than using metal beads, we surgically implant ~2.5-mm conical markers machined from carbide steel rods (following Jenkins et al. (1988) Science. 241: 1495-1498). These markers reliably anchor in the thin cortical bone around avian joints (Figure 2). We recorded birds in the W.M. Keck XROMM Facility while they performed a variety of behaviors. Six degree of freedom joint kinematics were extracted from bone animations using explicit Joint Coordinate Systems based on human biomechanical standards.
Our current understanding of avian bipedalism is deeply rooted in a 2-D paradigm. Kinematic and kinetic analyses are typically limited to flexion/extension, but birds and other so-called 'erect' tetrapods must routinely operate outside of the parasagittal plane. We are using marker-based XROMM to measure the 3-D motion and forces/moments in a chicken-like bird, the Helmeted Guineafowl (Numida meleagris), during maneuvering and steady locomotion (Figure 1). We are focusing on long-axis rotation (LAR) because soft tissue artifacts have previously prevented accurate measurement of this degree of freedom. Rather than using metal beads, we surgically implant ~2.5-mm conical markers machined from carbide steel rods (following Jenkins et al. (1988) Science. 241: 1495-1498). These markers reliably anchor in the thin cortical bone around avian joints (Figure 2). We recorded birds in the W.M. Keck XROMM Facility while they performed a variety of behaviors. Six degree of freedom joint kinematics were extracted from bone animations using explicit Joint Coordinate Systems based on human biomechanical standards.
Movie 1: Overhead animations of the guineafowl pelvic and hind limb bones during five maneuvers.
 Our first study (Kambic et al., 2014) examined maneuvering—specifically sidestepping, yawing, and turning (Movie 1)—although birds often performed a sequence of blended partial maneuvers (Movie 2). Long-axis rotation of the femur (up to 38°) modulated the foot's transverse position. Long-axis rotation of the tibiotarsus (up to 65°) also affected medio-lateral positioning, but primarily served to either reorient a swing phase foot or yaw the body about a stance phase foot (Figure 3). Tarsometatarsal long-axis rotation was minimal, as was hip, knee, and ankle abduction/adduction. Despite having superficially hinge-like joints, birds coordinate substantial long-axis rotations of the hips and knees to execute complex 3-D maneuvers while striking a diversity of non-planar poses.
Our first study (Kambic et al., 2014) examined maneuvering—specifically sidestepping, yawing, and turning (Movie 1)—although birds often performed a sequence of blended partial maneuvers (Movie 2). Long-axis rotation of the femur (up to 38°) modulated the foot's transverse position. Long-axis rotation of the tibiotarsus (up to 65°) also affected medio-lateral positioning, but primarily served to either reorient a swing phase foot or yaw the body about a stance phase foot (Figure 3). Tarsometatarsal long-axis rotation was minimal, as was hip, knee, and ankle abduction/adduction. Despite having superficially hinge-like joints, birds coordinate substantial long-axis rotations of the hips and knees to execute complex 3-D maneuvers while striking a diversity of non-planar poses.
Movie 2: A complex guineafowl maneuvering sequence. Animated bones are rendered relative to X-ray video, standard video, and a fixed pelvis in anterior view.

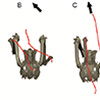 Our second study (Kambic et al., 2015) focused on symmetry in forward locomotion. During steady walking and running, right and left limbs are typically assumed to act out-of-phase, but with little kinematic disparity. However, outwardly-appearing steadiness may harbor previously unrecognized asymmetries. We found that guineafowl on a treadmill routinely yaw away from their direction of travel (Figure 4) using asymmetrical limb kinematics (Movie 3). Variation is most strongly reflected at the hip joints, where patterns of femoral long-axis rotation closely correlate to degree of yaw divergence. As yaw deviations increase, hip long-axis rotation angles undergo larger excursions and shift from biphasic to monophasic patterns. At large yaw angles, the alternately striding limbs exhibit synchronous external and internal femoral rotations of substantial magnitude. Hip coordination patterns resembling those used during sidestep maneuvers allow birds to asymmetrically modulate their medio-lateral limb trajectories and thereby advance using a range of body orientations (Figure 5).
Our second study (Kambic et al., 2015) focused on symmetry in forward locomotion. During steady walking and running, right and left limbs are typically assumed to act out-of-phase, but with little kinematic disparity. However, outwardly-appearing steadiness may harbor previously unrecognized asymmetries. We found that guineafowl on a treadmill routinely yaw away from their direction of travel (Figure 4) using asymmetrical limb kinematics (Movie 3). Variation is most strongly reflected at the hip joints, where patterns of femoral long-axis rotation closely correlate to degree of yaw divergence. As yaw deviations increase, hip long-axis rotation angles undergo larger excursions and shift from biphasic to monophasic patterns. At large yaw angles, the alternately striding limbs exhibit synchronous external and internal femoral rotations of substantial magnitude. Hip coordination patterns resembling those used during sidestep maneuvers allow birds to asymmetrically modulate their medio-lateral limb trajectories and thereby advance using a range of body orientations (Figure 5).
Movie 3: Guineafowl walking fast on a treadmill. Animated bones are rendered relative to standard video and from above to show pelvic yaw during steady locomotion.
References
Kambic, R.E., Roberts, T.J. and Gatesy, S.M. (2015). Guineafowl with a twist: asymmetric limb control in steady bipedal locomotion. Journal of Experimental Biology. 218: 3836-3844. Published article.
Kambic, R.E., Roberts, T.J. and Gatesy, S.M. (2014). Long-axis rotation: a missing degree of freedom in avian bipedal locomotion. Journal of Experimental Biology. 217: 2770-2782. Published article.
Jenkins, F.A., Jr, Dial, K.P. and Goslow, G.E., Jr. (1988). A cineradiographic analysis of bird flight: the wishbone in starlings is a spring. Science. 241: 1495-1498. Published article.

 Our current understanding of avian bipedalism is deeply rooted in a 2-D paradigm. Kinematic and kinetic analyses are typically limited to flexion/extension, but birds and other so-called 'erect' tetrapods must routinely operate outside of the parasagittal plane. We are using marker-based XROMM to measure the 3-D motion and forces/moments in a chicken-like bird, the Helmeted Guineafowl (Numida meleagris), during maneuvering and steady locomotion (
Our current understanding of avian bipedalism is deeply rooted in a 2-D paradigm. Kinematic and kinetic analyses are typically limited to flexion/extension, but birds and other so-called 'erect' tetrapods must routinely operate outside of the parasagittal plane. We are using marker-based XROMM to measure the 3-D motion and forces/moments in a chicken-like bird, the Helmeted Guineafowl (Numida meleagris), during maneuvering and steady locomotion ( Our first study (Kambic et al., 2014) examined maneuvering—specifically sidestepping, yawing, and turning (
Our first study (Kambic et al., 2014) examined maneuvering—specifically sidestepping, yawing, and turning (
 Our second study (Kambic et al., 2015) focused on symmetry in forward locomotion. During steady walking and running, right and left limbs are typically assumed to act out-of-phase, but with little kinematic disparity. However, outwardly-appearing steadiness may harbor previously unrecognized asymmetries. We found that guineafowl on a treadmill routinely yaw away from their direction of travel (
Our second study (Kambic et al., 2015) focused on symmetry in forward locomotion. During steady walking and running, right and left limbs are typically assumed to act out-of-phase, but with little kinematic disparity. However, outwardly-appearing steadiness may harbor previously unrecognized asymmetries. We found that guineafowl on a treadmill routinely yaw away from their direction of travel (

